Nitrogen Cycle Animatic no sound from Erin Siegel on Vimeo.
In addition, here's the script and some rough animations for the film. The script needs some more refinement, Myrna will be checking it for accuracy and making sure all the details we need are there. The rough animations here are just some cloneable subjects I'll use to fill up the background. If I have the time after all the rough animation is finished, I'll paint these first.
Earth, it is our home and a precious resource. Everything life needs to thrive is found on this tiny blue speck in space. Some of the most necessary elements for our survival can be found not on Earth’s surface, but in its atmosphere.
All around you see these twinkling blue dots. They represent nitrogen. Nitrogen is a vital element, it makes up the backbone of DNA, chains of molecules called amino acids, and proteins which make up living things. That’s why nitrogen is important to every organism on earth, including you and me. Now even though nitrogen is all around us, most living things can’t use it in its purest form. It must be fixed or bound to other molecules. Sometimes lightning strikes fix nitrogen, but the vast majority of fixed nitrogen is made on land and in the sea.
Here amongst the seagrass we happen upon a microscopic world where diazotrophs, or nitrogen-fixing bacteria, live. They use pure nitrogen to sustain themselves, releasing ammonium as a byproduct. The ammonium is then used by nitrifying bacteria and combined with oxygen to produce nitrites and nitrates. Other microorganisms and plants absorb the nitrates, which is passed up through the food chain to animals such as this fish and fish eaters.
When an organism excretes or dies, the nitrates from its body are used by decomposer bacteria. They convert the nitrates in ammonium, which then can filter deeper into the oxygen-less organic layers of soil. The denitrifying bacteria which thrive in this environment change the ammonium back into nitrogen and another compound called nitrous oxide. The nitrogen and nitrous oxide then return to the atmosphere.
The nitrogen cycle is a delicate balance. With enough nitrogen fixed and exchanged throughout the environment, the ecosystem is fine. Too little nitrogen and growth is severely inhibited. Only with the right amount of nitrogen can an environment thrive.
The same principle applies in agriculture, where crops depend on the right amount of nitrates to grow and sustain a small population of people. But by the beginning of the industrial age, there wasn’t nearly enough to feed the global population boom. A dire situation, no one knew how to pump more nitrogen into the system. Then in 1909, Haber (the Bosch) discovered a way to convert methane into ammonia to fertilize crops. This chemical fertilization was so effective, it created an explosion in agricultural production. Now there are more vegetables, fruits, grains and consequently meat to feed growing industrialized nations.
But chemical fertilization taxes the delicate nitrogen cycle. When the excrement of animals and excess fertilizer washes into streams and rivers, it flows back into the marine ecosystem and plays havoc with habitats. With so much ammonium and nitrates to absorb, sea grass and marine bacteria grow out of control, choking the water and leaving less room for other species. In the process, all free oxygen is used up, suffocating any oxygen-breathers left. Both plants and animals begin to die, coating the sea floor with their decaying bodies.
However something worse, much worse, than this local ecological disaster is taking place. Because there is so much organic build up, what little oxygen is left is prevented from reach the lower layers of soil. Denitrifying bacteria, which prosper in this anoxic environment, are able to produce far more nitrogen and nitrous-oxide than previously. This excess of nitrous oxide rises high into our atmosphere, eating away at the ozone layer. And at still higher altitudes, it acts as a potent greenhouse gas X times more powerful than carbon dioxide. With so much nitrous oxide, eventually…[earth heats, lights go out]
So how do we reverse it? With a little brain power. By collecting the excess fertilizer before it reaches the ocean, we can dramatically reduce the amount of nitrous oxide generated at sea. Special algae mat filters absorb the nitrate runoff, and when the mats are completed covered, the algae can then be used by agriculturalists as a brand new fertilizer, just as effective and more environmentally sound than chemical treatment.
Now we can feed the nation and preserve the world for our Nitrogen-knowing future.
And here's the rough animations:
A fish
A diazotroph, or nitrogen-fixing bacteria
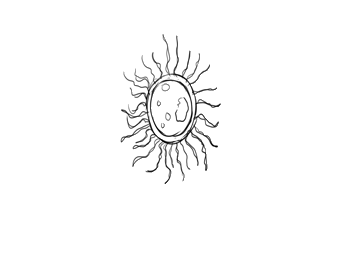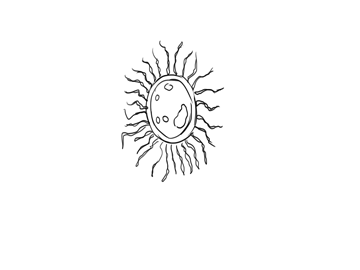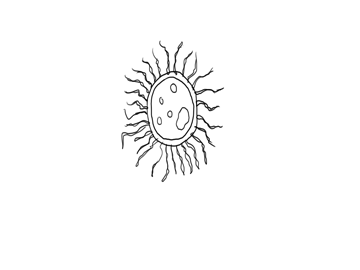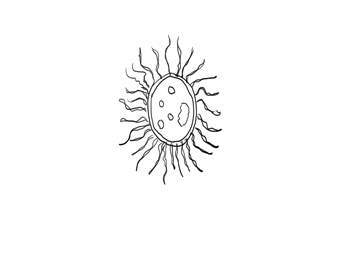
A decomposer bacteria
some plant
Blade of seagrass
Clump of seagrass.

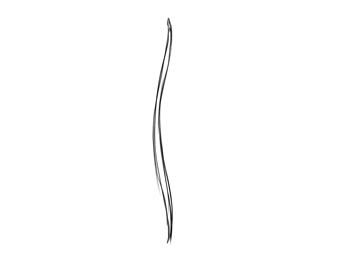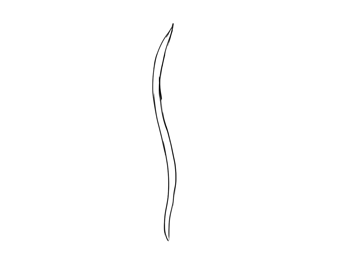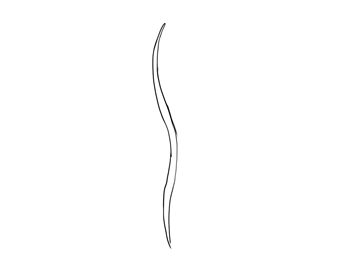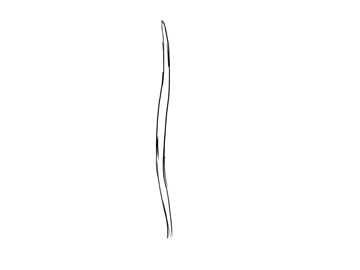
No comments:
Post a Comment